Regulatory Support Services
Our Unrivaled Expertise in Regulatory Services for Clinical Trials.
At YouV, we leverage decades of regulatory expertise to ensure the seamless and efficient execution of your clinical trials. Our specialized teams deliver customized solutions that accelerate the approval process while ensuring compliance with international standards.

End-to-End Regulatory Services for Your Trials.

- Prepare, quality check and submit regulatory documents/packages to the Sponsor, IRB/IEC/EC and Data safety boards or equivalent committees, as applicable
- Collaborate with the clinical research team to ensure submission materials are current and compliant with regulations.
- Maintain regulatory documentation, including consent forms, protocols, 1572s or equivalent, CVs, licenses, investigator brochures, recruitment materials, safety reports, and submission forms (and translations, when applicable)
- Oversee study start-up activities, including regular reporting and communication with stakeholders, prior to the release of the first shipment of IP to investigational sites.
- Monitor the approval status of open studies by conducting timely reviews.
- Offer guidance and training to investigational site research staff to ensure compliance with regulations.

- Stays up-to-date with federal, state, and institutional guidelines related to clinical trials.
- Continued guidance and training research staff to ensure compliance with regulations governing clinical research studies and any applicable amendments.
- Act as a liaison between Sponsors, CRO, IRB, and Investigators, ensuring continuity of service and prompt resolution of issues.
- Maintain master files of all regulatory-related documents/sections.
- Assist in the submission of amendments to approved documents and required reporting.
- Ensure proper closure and compliance with all relevant guidelines prior to site closeouts
- Final Reporting: Prepare and submit final study reports to regulatory authorities, including summarizing the study’s findings, safety data, and any deviations from the protocol
- Regulatory Submissions: Complete and submit all necessary documents for study closure, such as final safety reports, clinical study reports, and any required notifications to ethics committees or institutional review boards (IRBs).
- Data Archiving: Ensure all study data, including patient records and regulatory documents, are archived according to regulatory requirements, employing secure storage systems for long-term data retention.
- Audit Preparation: Prepare for potential audits by regulatory bodies, including organizing all study documentation, ensuring all records are complete and accurate, and addressing any outstanding issues.
- Regulatory Communication: Communicate with regulatory authorities to confirm the study’s closure and address any follow-up questions or requirements.
- Publication Support: Assist with the preparation and submission of study results for publication in scientific journals, to ensure that all regulatory requirements for transparency and reporting comply.
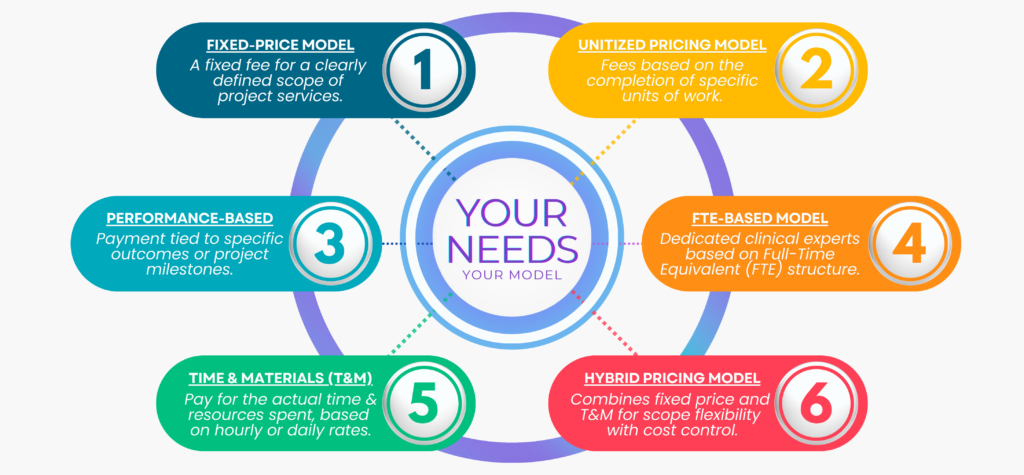
Tailored FSP Models for Regulatory Support Services.
At YouV, we tailor flexible FSP models to meet the unique needs of your clinical trial. Our comprehensive regulatory support ensures compliance with international and national laws, customized to your investigational product and study requirements. Explore our models below:
Our Expertise
Achieve Excellence in Regulatory affairs.
Let’s simplify your regulatory journey. Contact us today to learn more about our Regulatory Affairs Services!