Careers
Let’s Make Work Meaningful Together!
We believe that alternative thinking gives rise to ideas. Adopting smart work, we put efforts into creating human-centric processes which critically focuses on teamwork, co-creation and co-execution.
As part of YouV, you can expect to learn skills beyond your designation/role. YouV will provide you with an unparalleled exposure, and become an essential stepping stone that will add great value to your career. Not just exposure, training, and a positive work environment, you will also gain valuable learning from exceptional people around you.

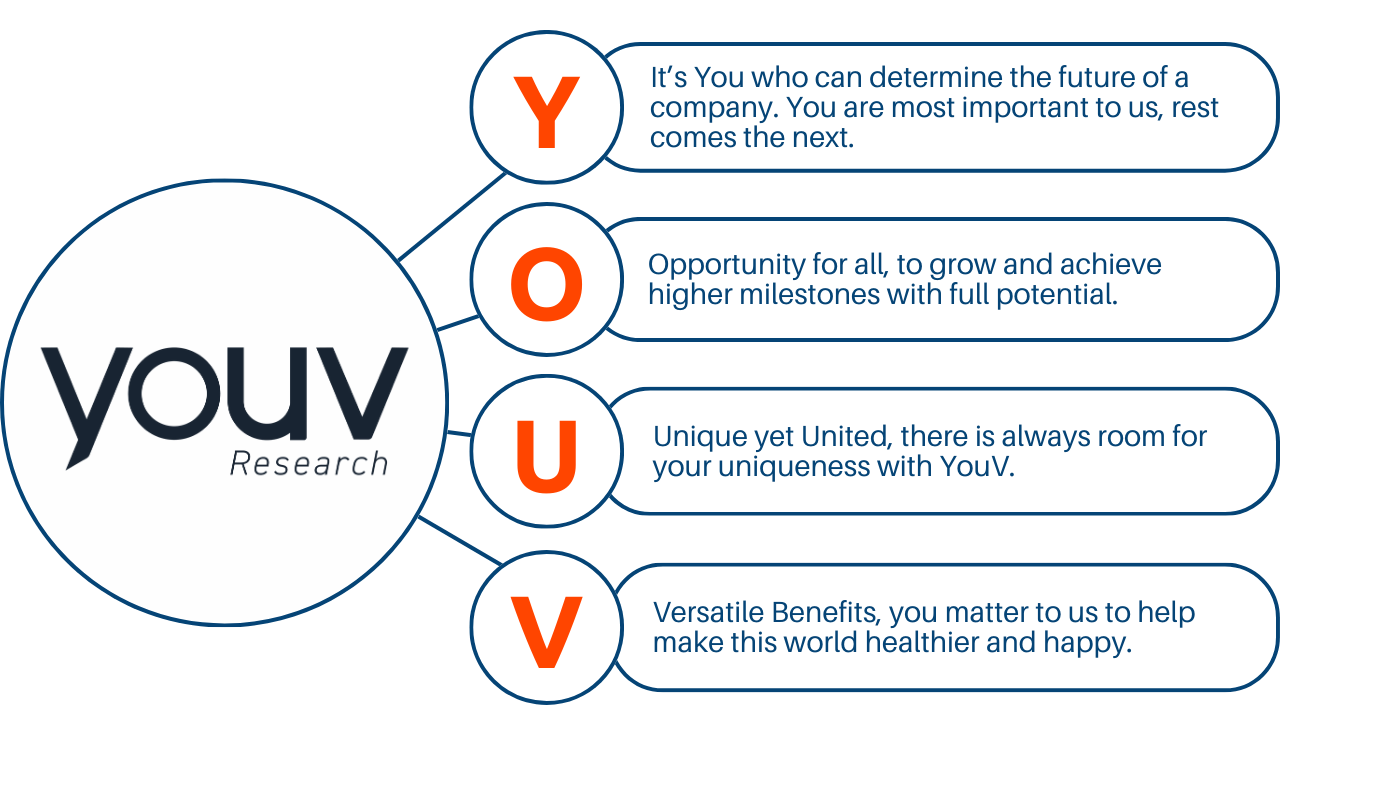
Our Values & Culture.
Get ready to immerse in an environment which bespeaks a unique flair of warmth and positivity. From enjoying a great sip of coffee to a fun happy hours to some serious work, we have it all. Get to know us what we mean by the collaborative, open-door and inclusive environment.
Start Your Career @YouV
Current Openings:
Biostatistics - Senior Director
Qualification:
Education level:
- Master’s or Ph.D. degree in Statistics, Biostatistics, or a related field
Experience:
- Minimum of 14 years of progressive experience in Biostatistics within the pharmaceutical, biotechnology, or Contract Research Organization (CRO) industry, with a focus on biostatistics.
- Vast experience in leading biostatistics departments within the pharmaceutical industry or CRO
- Expertise in statistical analysis, study design, and regulatory requirements
- Strong leadership and strategic planning abilities
- Proven ability to establish and maintain relationships with key stakeholders.
Job Description:
- Oversee the overall performance and operations of the biostatistics department
- Drive the development and execution of departmental strategies and goals
- Foster a culture of excellence, innovation, and collaboration within the team
- Engage with senior management on strategic initiatives and departmental goals
- Represent the organization in external forums and industry conferences
Responsibilities and Duties:
- Provide executive-level leadership to the biostatistics department.
- Drive the development and execution of a comprehensive biostatistics program.
- Establish strong partnerships with clients and key stakeholders.
- Mentor and develop senior statisticians and biostatisticians.
- Represent the organization in external forums and industry conferences.
- Stay informed about emerging trends and advancements in biostatistics and clinical research.
Biostatistics - Director
Qualification:
Education level:
- Master’s or Ph.D. degree in Statistics, Biostatistics, or a related field
Experience:
- Minimum of 12 years of hands-on experience in Biostatistics within the pharmaceutical, biotechnology, or Contract Research Organization (CRO) industry.
- Extensive experience in leading statistical teams and overseeing clinical trial research.
- Strong knowledge of regulatory guidelines and industry standards
- Proven ability to drive innovation and implement best practices in biostatistics.
- Excellent communication and presentation skills, with the ability to influence stakeholders at all levels.
Job Description:
- Provide strategic leadership in biostatistics, overseeing multiple projects and teams.
- Establish and maintain strong client relationships and collaborations.
- Drive process improvement initiatives and implementation of best practices
- Contribute to business development activities, including proposal development and client presentations.
- Stay updated with industry trends and advancements in statistical methodologies.
Responsibilities and Duties:
- Provide strategic direction and leadership to the biostatistics department.
- Manage resources, budget, and timelines for multiple projects.
- Ensure compliance with regulatory requirements and industry standards.
- Engage with clients and key stakeholders to understand their statistical needs.
- Identify opportunities for process optimization and innovation in biostatistics.
- Foster a culture of excellence, innovation, and collaboration within the team.
Biostatistics - Associate Director
Qualification:
Education level:
- Master’s or Ph.D. degree in Statistics, Biostatistics, or a related field
Experience:
- Minimum of 10 years of experience in Biostatistics within the pharmaceutical, biotechnology, or CRO industry.
- Significant experience in leading biostatistics teams in the pharmaceutical industry or CRO
- Demonstrated leadership in statistical analysis, study design, and regulatory submissions.
- Proven track record of successful project management and client engagement
- Excellent strategic thinking and decision-making skills
Job Description:
- Lead and manage the biostatistics department, providing strategic direction and oversight.
- Drive the development and implementation of innovative statistical methodologies.
- Engage with clients and stakeholders to understand their statistical needs and requirements.
- Ensure efficient project execution, meeting timelines and quality standards.
- Foster a culture of continuous learning and professional development.
Responsibilities and Duties:
- Lead and supervise a team of statisticians and biostatisticians.
- Set departmental goals and objectives, aligning with organizational strategies.
- Monitor project progress, timelines, and budget utilization.
- Establish and maintain effective communication channels with clients and stakeholders.
- Evaluate and implement process improvements for increased efficiency and quality.
- Stay updated with industry trends and advancements in statistical methodologies.
Biostatistics - Associate Statistician
Qualification:
Education level: Bachelor’s degree in Statistics, Biostatistics, or a related field
Job Description:
- Assist in conducting statistical analysis for clinical trials.
- Perform data cleaning and validation.
- Generate summary tables, figures, and listings.
- Assist in the development of statistical analysis plans and study protocols.
- Collaborate with cross-functional teams to ensure data quality and integrity.
- Contribute to the interpretation of study results.
- Stay updated with relevant regulations and industry best practices.
Responsibilities and Duties:
- Conduct statistical analysis under the guidance of senior statisticians.
- Perform data management tasks, including data cleaning and transformation.
- Assist in generating analysis datasets and statistical outputs.
- Collaborate with programmers and data managers to ensure data quality and consistency.
- Contribute to the development and review of study documents and regulatory submissions.
- Participate in departmental meetings and training programs.
- Stay informed about emerging statistical methodologies and industry trends
Biostatistics - Manager
Qualification:
Education level:
- Master’s degree or Ph.D. in Statistics, Biostatistics, or a related field
Experience:
- Minimum of 8-10 years of progressive experience in Biostatistics, including leadership roles, within the pharmaceutical, biotechnology, or CRO industry.
- Extensive experience in statistical analysis and clinical trial research
- Strong leadership and management skills
- Excellent communication and presentation abilities
- Ability to collaborate with cross-functional teams
Job Description:
- Manage and supervise a team of statisticians and biostatisticians.
- Oversee statistical analysis and reporting activities for clinical trials.
- Provide guidance and support in study design, sample size estimation, and statistical methodologies.
- Collaborate with cross-functional teams to ensure timely and accurate deliverables.
- Maintain compliance with regulatory guidelines and industry standards.
Responsibilities and Duties:
- Manage and allocate resources within the biostatistics team.
- Ensure adherence to quality and regulatory standards in statistical analysis.
- Collaborate with project teams to develop statistical analysis plans.
- Oversee the preparation of statistical deliverables for regulatory submissions.
- Provide guidance on complex statistical issues and study interpretations.
- Foster a culture of continuous learning and professional development within the team.
Biostatistics - Senior Statistician
Qualification:
Education level:
- Master’s or Ph.D. degree in Statistics, Biostatistics, or a related field
Experience:
- Minimum of 5-7 years of industry experience as a Statistician within the Life Sciences industry or Contract Research Organization (CRO).
- Significant experience in designing and analyzing clinical trials.
- Expertise in statistical software and advanced statistical methods
- Thorough understanding of regulatory guidelines and industry standards
- Proven leadership and project management skills
- Strong publication record and ability to mentor junior statisticians.
Job Description:
- Oversee statistical analysis activities across multiple clinical trials.
- Provide expert statistical guidance to project teams and stakeholders.
- Develop innovative statistical methodologies and contribute to research publications.
- Lead the preparation of statistical deliverables for regulatory submissions.
- Mentor and supervise junior statisticians, providing training and professional development opportunities.
Responsibilities and Duties:
- Lead and manage statistical analysis projects
- Conduct complex statistical analyses and critical data interpretations.
- Guide the development and review of study protocols and statistical analysis plans.
- Ensure compliance with regulatory requirements and industry standards.
- Collaborate with cross-functional teams and external partners.
- Stay updated with emerging statistical methodologies and industry trends.
- Participate in business development activities, such as proposal development and client presentations.
Biostatistics - Statistician I
Qualification:
Education level:
- Master’s degree in Statistics, Biostatistics, or a related field
Experience:
- Minimum of 1-2 years of industry experience as a Statistician within the Life Sciences industry or Contract Research Organization (CRO).
- Experience in statistical analysis using SAS or R
- Familiarity with clinical trial protocols and statistical analysis plans
- Strong data interpretation and presentation skills
- Ability to work collaboratively in a team environment.
Job Description:
- Assist in conducting statistical analysis for clinical trials.
- Perform data cleaning and validation.
- Generate summary tables, figures, and listings.
- Assist in the development of statistical analysis plans and study protocols.
- Collaborate with cross-functional teams to ensure data quality and integrity.
- Contribute to the interpretation of study results.
- Stay updated with relevant regulations and industry best practices.
Responsibilities and Duties:
- Perform statistical programming and analysis tasks.
- Create and maintain analysis datasets and statistical outputs.
- Collaborate with data managers and clinical teams.
- Review and contribute to study documents and regulatory submissions.
- Stay updated with statistical methodologies and industry best practices.
- Participate in project meetings and provide statistical insights.
- Support the development and maintenance of SOPs related to biostatistics.
Biostatistics - Statistician II
Qualification:
Education level:
- Master’s or Ph.D. degree in Statistics, Biostatistics, or a related field
Experience:
- Minimum of 2-3 years of industry experience as a Statistician within the Life Sciences industry or Contract Research Organization (CRO).
- Extensive experience in statistical analysis using SAS, R, or other statistical software.
- In-depth knowledge of clinical trial design, protocols, and regulatory requirements
- Excellent problem-solving and critical thinking abilities
- Strong written and verbal communication skills
Job Description:
- Lead statistical analysis activities for clinical trials.
- Develop and review statistical analysis plans and study protocols.
- Ensure data integrity and quality through validation and quality control procedures.
- Provide statistical interpretation of study results.
- Contribute to process improvement initiatives and standardization efforts.
- Collaborate with study teams and contribute to scientific discussions.
Responsibilities and Duties:
- Design and implement statistical analysis plans.
- Perform complex statistical analyses using advanced methodologies.
- Validate analysis datasets and statistical output.
- Provide statistical interpretation of study results.
- Contribute to process improvement initiatives and SOP development.
- Mentor junior statisticians and provide guidance on statistical methodologies.
- Stay informed about emerging statistical techniques and industry guidelines.

